Week Four – MisCELLaneous Tasks
March 31, 2023
Hola. This week, I largely did more of the usual (passaging cells and reading articles for my senior project). Unfortunately, one of the cell types did not survive again. I passed them correctly, but something must have happened to cause them to die prematurely. So tricky!
Despite that mishap, I did have fun helping Purva with her other work. I labelled test tubes, prepared a milk solution, made cell media, and found formaldehyde in the lab. This may sound disconnected, because it is: Purva manages a bunch of experiments at once. The test tubes were for a series of centrifugations of mouse interstitial fluid: the first set was spun at 2,000 g for 45 minutes at 4℃, the other that and also, afterwards, 10,000 g for 45 minutes at 4℃. This separates out certain components, like apoptic bodies and endosomes, leaving the extracellular vesicles of interest to Purva’s research.
The milk solution was for another western blot. A mixture of non-fat milk powder and wash buffer (a corrosive buffer agent known as tris, salt, detergent, and water), the lactescent liquid saturates excess protein-binding sites in order to enhance the sensitivity of the final gel. The cells I work with cost $541.00 per vial from the American Type Culture Collection (ATCC). The new microscope that came in apparently costs $30,000. So let me say, I was surprised when I heard the people at CAPMM use something as cheap and pedestrian as milk powder for their experiments. Goes to show that science is everywhere, and to appreciate the rich variation of complex proteomic interactions that undergird our daily lives, from digestion to exercise.
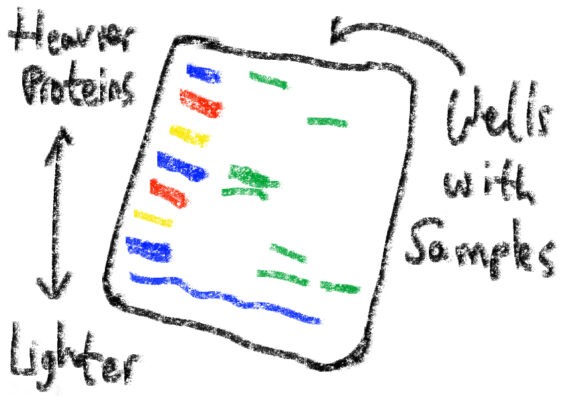
Western blot gel. The leftmost column contains a marker for different weights of protein.
For the cell media, I poured 150 mL of the raw media into a filter and added 15 mL of fetal bovine serum (FBS). FBS is the blood plasma (minus clotting factors) of bovine fetuses which contains electrolytes, antibodies, hormones, lipids, growth and attachment factors, and other miscellaneous components necessary for the (for some reason, murine) cells to grow correctly. With a continuous pump of air for a minute, the mixture passes through the filter and neatly into a flask, which then chills in the refrigerator until it is needed. The method of manufacture of FBS sounds satanic, but I suppose a comparable replacement for the purposes of accurate-to-life cell culture is nearly impossible to create synthetically.
Lastly, the formaldehyde: I found it in the bottom-right side of the leftmost cabinet under a fume hood, next to formic and glutamic acid. Purva diluted it to 3% in phosphate-buffered saline (PBS) and pipetted 200 μL over the cells of interest, in order to fix them for later imaging using immunofluorescence.
Reflect that this is science: many measured steps leading up the mountain of discovery. Happy March!
