Week One: All About the Ducks
March 8, 2024
I have officially finished my first week at MITRE! My week started with lots of employee orientation activities. The bulk of those activities were introductory presentations and training videos. After receiving my badge and laptop (my arm is sore from carrying it around), I received a tour of the entire campus. There are four buildings to navigate (and get lost in), but I think I’ve done a pretty good job of finding my way around so far. Then, I met with my on-site mentor, Dr. Joe Roberts (Joe), and met my other coworkers who will work with and guide me throughout the next three months. They are wonderful and have been so incredibly kind and welcoming to me.
Additionally, I toured the Bio-Nanotechnology lab I’ll work in (my “home base”). Joe and I got to eat free from the cafeteria on Monday; I personally think the dessert is especially great. While I’m still getting used to all the websites and procedures, there’s already a lot of other things I’m excited about. Waking up early is becoming easier, and I take advantage of the free tea and coffee provided. MITRE also has these fancy elevators that I’ve never seen before – instead of pressing a button for up and down, you select your desired floor on a touch screen, and it redirects you to one of six carts. The last time I was in an office building was with my mom during “Bring Your Child to Work Day,” so it’s surreal that I’m back in one for this internship.
Other than orientation activities, I spent time familiarizing myself with the project I’m interning on and the main technique used in the lab: Cyclic Voltammetry (CV). I read an introductory guide to CV my coworkers shared with me and researched the basics of CV. CV is the technique I mentioned in my abstract and blog post last week. The current of an electrochemical cell is measured as an external power source – potentiostat – manipulates the cell potential (voltage) applied. Thus, we can observe the electrochemical behavior, or reduction and oxidation (the gain and loss of electrons, respectively), of a molecular species.
In this case, the molecule ortho-phenylenediamine (o-PD) is electropolymerized (joined together by electricity) on the electrode surface. PFAS molecules interact with the o-PD, causing a decrease in current that can be associated with the presence/absence of PFAS. The sensors are very small, rectangular gold-plated electrodes called screen printed electrodes (SPE); the chemistry only happens on the bottom half of it. I’ve included a simplified diagram of an SPE below for visual reference.
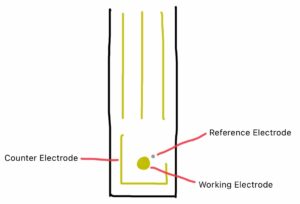
(Fiona Xu) (ACS Publications – American Chemical Society)
One of my coworkers took me inside the lab to show me how to use CV to perform something called Molecularly Imprinted Polymer (MIP) acid fabrication. In simple terms, it’s basically preparing the area on the sensor (the gold circle) PFAS will interact with. I then saw the characteristic CV duck curves (shown above) on the lab computers. As the cell potential is manipulated or the o-PD is reduced and oxidized, the current peaks on the graph are explained by the Nernst Equation, which I learned in my AP Chemistry class (yay for real-life applications). Depending on the conditions of the CV experiment, some curves look more duck-shaped than others. I completed lab training on Thursday, so hopefully I’ll start conducting experiments in the lab next week.
As for my independent research, I started skimming through and compiling sources about the Potomac River’s general properties. I’m also in the process of researching the history and current situation of PFAS contamination in the Potomac River. Before I research the applicability of these sensors to Potomac River water samples and per Joe’s suggestion, I need to understand how severe PFAS contamination in the Potomac is first and whether it’s a persisting issue. This was definitely a memorable first week.
Thanks for reading all the science-speak,
Fiona
Citations
Elgrishi, N., Rountree, K., McCarthy, B., Rountree, E., Eisenhart, T., & Dempsey, J. (2017). A Practical Beginner’s Guide to Cyclic Voltammetry. Journal of Chemical Education, 95(2), 197–206. https://doi.org/10.1021/acs.jchemed.7b00361

Leave a Reply
You must be logged in to post a comment.