Week 5: Plants!
May 1, 2024
Hey y’all! So glad you could join me for the fifth week of my blog!
Firstly, I am extremely grateful to announce that Matias and I were able to sow the final test group of alfalfa plants on Thursday, April 25th. In the last blog, I outlined my process of brainstorming variables to control for this final group; my focus for this week shifted towards implementing these controls in the greenhouse environment.
It is the perfect time for alfalfa growth right now! Temperatures in Cold Spring Harbor are becoming more agreeable to both plants and people, while the photoperiod (ratio of light to darkness time each day as sensed by crops) indicates to many seeds that it is the ideal season for germination. These factors encouraged my decision to plant this week, as well as the fact that I will have around 40-50 days of growing time before collecting biomass samples (and more sets of data!) for my final presentation.

Earlier in the week, I prepared a few other updates to my experimental design that I’d love to share. To predict the approximate size of the roots that will grow in the final trial, I uprooted two plants from Week 1 to see if we could downsize our area of the potting inserts. The initial trays were around six square inches– which I downsized to two based on the larger plants (Figure 1). Additionally, my plan to use a random block design from Weeks 3 & 4 came to fruition as I discussed what a reasonable number of experimental replicates for each treatment would be with Matias.
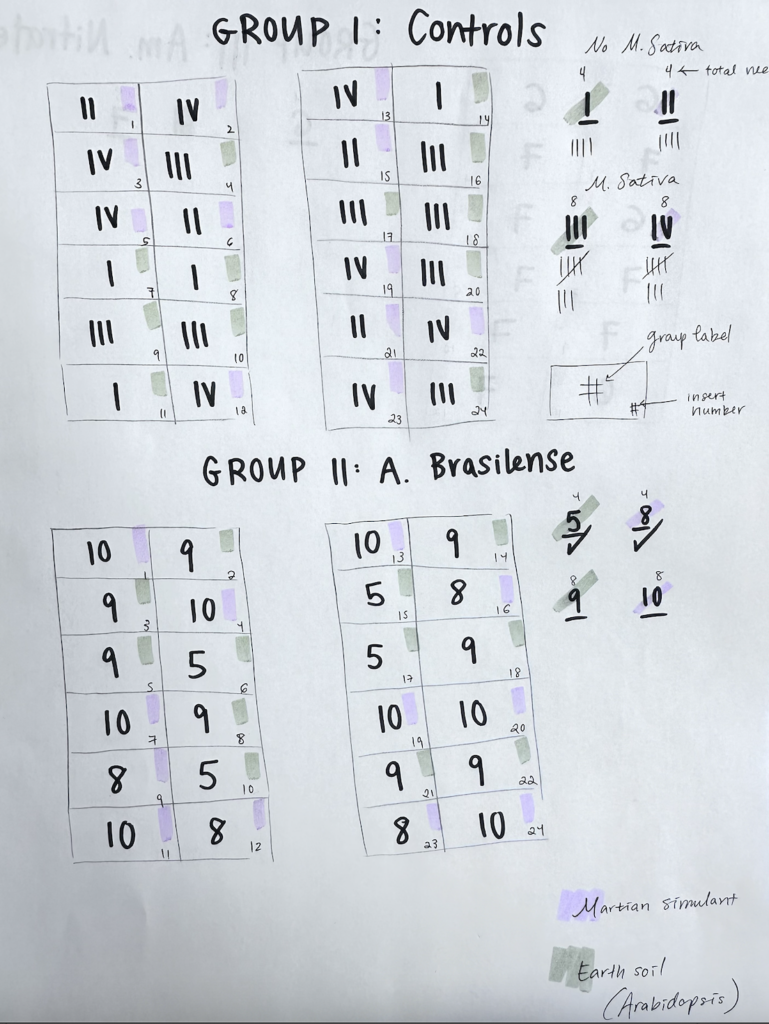
The least amount of replicates recommended by Matias was three, and as such, I decided to include four pots per group without alfalfa, and eight pots per group with an alfalfa plant. But before randomizing the positions of each potting insert, I had to consider a constraint of the greenhouse flats: treatment groups could not be mixed in the same flat.
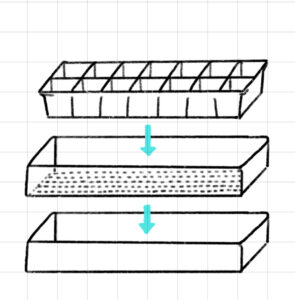
As visualized in Figure 2, Each flat comes with a solid tray at the bottom, followed by a drainage tray, and then finally inserts at the top. According to Prof. Jackson, any treatments applied to the plants that grow in a given flat will migrate across the solid tray, contaminating all groups on the flat. Because of this, I had to randomize the layout of this experiment categorized by treatment type: None (Negative Control), Azospirillum Brasilense (Biofertilizer, Experimental), and Ammonium Nitrate (Chemical Fertilizer, Positive Control). These groups were organized and numbered to be sown. (Figures 3 & 4)

After deciding on the design layout, we organized and built the greenhouse flats to completion, filling the various groups according to their intended soil-type, plant amount, and fertilizer-type. Appropriate safety equipment was used during the planting, as the Martian soil simulants are extremely fine and possibly irritating, but opening up the astro-soils was an incredibly rich experience. We watered each group after adding the soil, and the simulants were highly sand-like, mimicking the dusty, but dense nature of non-domesticated growing environments like Mars. (Figure 5)

What Next?
In the next few weeks, I plan to inoculate (treat with bacteria) the reserved Azospirillum flats. For the sake of accuracy, Matias and I will verify the CFU (colony forming units) of the Azospirillum Brasilense projected by its spec sheet. CFU are a good measure of the amount of bacterial colonies contained by a given freeze-dried bacteria after rehydration. We intend to grow a collection of ~100 CFU, which will be counted after being cultured in a LB (Lysogeny Broth – A medium of nutrient-rich agar used for growing bacteria) plate *without antibiotics.
Regarding research, I will be assessing whether Azospirillum Brasilense can be “marked” (attached to) a fluorescent protein that will make it glow under imaging software. If the bacteria responds well historically to GFP (green fluorescent protein) or RFP (red fluorescent protein), then we may be able to visualize the movement of the bacteria around the plant’s root system for the final project. On another note, I will be reading a few papers from CSHL postdoc Penelope Lindsay, who is well-versed in the relationships between bacteria, fungi, and plants.
Thanks for tuning in, and I look forward to updating as our plants grow!
Reader Interactions
Comments
Leave a Reply
You must be logged in to post a comment.

Yuna, I’m so excited that the final alfalfa plant test group went well! I’m glad to hear that you are effectively responding to the concerns brought up by Professor Jackson, strategically planning the experiment layout by treatment type in order to account for the design of the greenhouse flat. I also love that you included Figures 3 and 4 in this post. It gives us a better view of the hard work you are doing by helping us visualize your planning. I’m excited to hear how your inoculation goes! I’m curious if the visualization of bacteria movement around the plant’s root system using the imaging software you mention at the end can be recorded and shared in your blog? That would be fascinating to see!
Thank you so much Sam! I will definitely be sharing the images that we collect of the bacteria’s movement, the hope is that we can document the relationship between the root system, or “rhizosphere,” and the nitrogen fixing colonies within our soil. I can’t wait to update how it goes!