Week 6: Solutions, Equations, and Germination
May 7, 2024
Hi-ya! Welcome back to the sixth blog of my Senior Project!
To recap, last week I was able to fill and sow my main alfalfa groups, which will be stored in the greenhouse until they reach maturity (~40-50 days). These plants and the biomass samples that can be collected from them will serve as the exhibit for many experiments to evaluate their overall health in the future. Lots of hands-on lab work going on this week, so I’ll jump straight into the important stuff.
Firstly, our plants got their initial dose of fertilizer this week! Thursday began promptly with checkups around the greenhouse, a routine I plan to keep constant throughout the remainder of this project. When designing my experimental plan, I had ensured that the plants had at least one week to adjust to the greenhouse environment, after which I would begin to inoculate the inserts of the Azospirillum labeled groups. Inoculation (in this instance) refers to the process of introducing a species of bacteria to a given environment, and we decided to perform it “in-furrow,” meaning the soil around each seed was treated with a bacterial solution. Other methods include spraying the plant, dipping the seeds, dipping the roots, and many more.
Fertilization
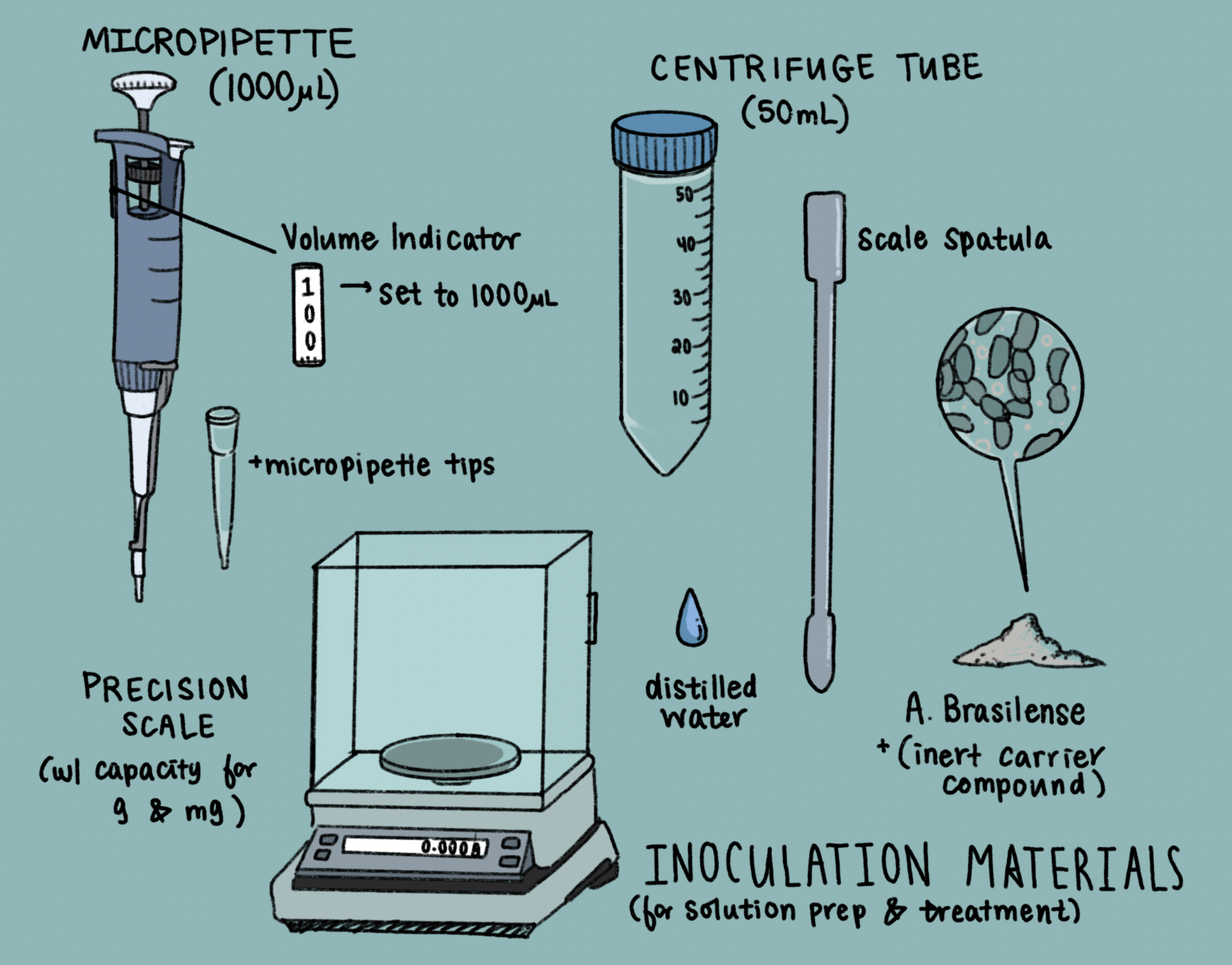
In anticipation of the bacterial treatments, I calculated several variables we needed to prepare an appropriate concentration for the individual inserts. The recommended doses for Azospirillum began at 1 Gallon per every 100 ft2, a metric appropriately scaled to handle large agricultural plots. As each of my inserts have an area of 4.15 square inches, I converted the concentration to a more agreeable unit: 260 μL (microliters) per in2. The powdered Azospirillum (Figure 1) that we bought comes in an inert carrier, which holds 10^7 CFU/Gallon; CFU is “colony forming units,” indicating the number of bacterial colonies that will grow in a given amount of solution. To calculate the required volume we needed to fertilize each plot, I multiplied the 260 μL concentration by the area, yielding a dose of 1079 μL/insert.

With a total of 24 inserts requiring our biofertilizer, Matias and I prepared a solution with 75mg/50mL (the same concentration calculated according to the recommended treatment). Azospirillum powder was weighed via precision scale, and mixed in solution with distilled water. (Figure 2)

After preparing the materials for inoculation (Figure 3 – drawing), we drove up to the greenhouses to assess and treat the plant groups from last week. I was happy to see that plants were successfully germinating in the control and earth experimental groups (Figure 4).

Greenhouse Update
On a more depressing note– MGS1-planted seeds, however, were lagging behind the earth groups and did not germinate (boo!). A scary sight for sure, but not at all unpredictable due to the ruthless composition of the simulant. I hypothesize that this stunt in growth was likely due to the density of the soil: when prodded with a glove, the surface was solidified, mimicking a compact feel, like concrete. Optimistically the problem is a lack of water retention by the Martian simulants. MGS-1 possesses a more sand-like quality that causes water to evaporate. Alfalfa seeds, or any seed for that matter, require a baseline humidity in order to break the dormant phase they naturally fall into. Plant germination requires several factors, like sunlight, even temperatures, but also water –something evidently absent from the cake-y textures of the Martian soil groups, but abundant in each earth tray.
This setback for germination in my experimental plants was certainly alarming, but I am staying optimistic that the plants also lack the required macronutrient Nitrogen (and its derivatives). Inoculating with Azospirillum is designed to introduce the missing nitrogenous compounds and stimulate plant growth responses. So with the previously prepared bacteria solution, I micropipetted 1079 μL of treatment into the non-control and negative control groups.
After finishing inoculation, we left instructions for the greenhouse staff to keep the Martian groups wet throughout the growth cycle. They will bottom-water the flats every 2 (Mars) to 3 (Earth) days.
Finally, As mentioned last week– I also prepared a solution of A. Brasilense to incubate in LB agar. After taking a 10 mg measurement of the bacteria and creating a stock solution in 1000 μL, a serial dilution was performed, and 1 μL of the stock was added to 99 μL. The resulting solution was 100 μL to be streaked (spread evenly across) an agar plate without antibiotics (Figure 5). The test was performed twice across 2 plates (total 200 μL) and set to incubate at 37°C for a week.

What’s next?
With the final project approaching in a month and a half, next week I will be discussing a literature review of bacteria movement visualization techniques. Our intention is to develop a protein marking system to compile imagery from the activity of Azospirillum as it colonizes the roots of alfalfa– this will be integral in describing the importance of a healthy root system, and showing how nitrogen fixers interact with the native microbiome of plants underground.
See you next week, ciao!
Reader Interactions
Comments
Leave a Reply
You must be logged in to post a comment.

Wow Yuna! This is such an insightful blog post! It’s great that you were able to treat your plants- hopefully next week many more seeds will sprout as well!