Week 7: Microscopy, LB, and Wall-E
May 14, 2024
Hi plant people! Thanks for joining me for the seventh week of my project update!
Speed recap:
Last time at the lab, we treated the greenhouse flats with and without alfalfa by inoculating them using a measured bacterial solution. Additionally, our Mars plants were observed to be lagging behind their terrestrial counterparts in germination time; and finally, I prepared a plate to grow an Azospirillum colony test separately, which I will count by hand this week.
The timeline for our project was conveniently time-generous this week, so my focus shifted primarily to researching plausible concepts for use with the aforementioned transgenic bacteria (referenced briefly, Week 2). Before diving into the wet-lab work, I’d like to introduce some relevant background from bacterial genetic engineering.
Agrobacterium:
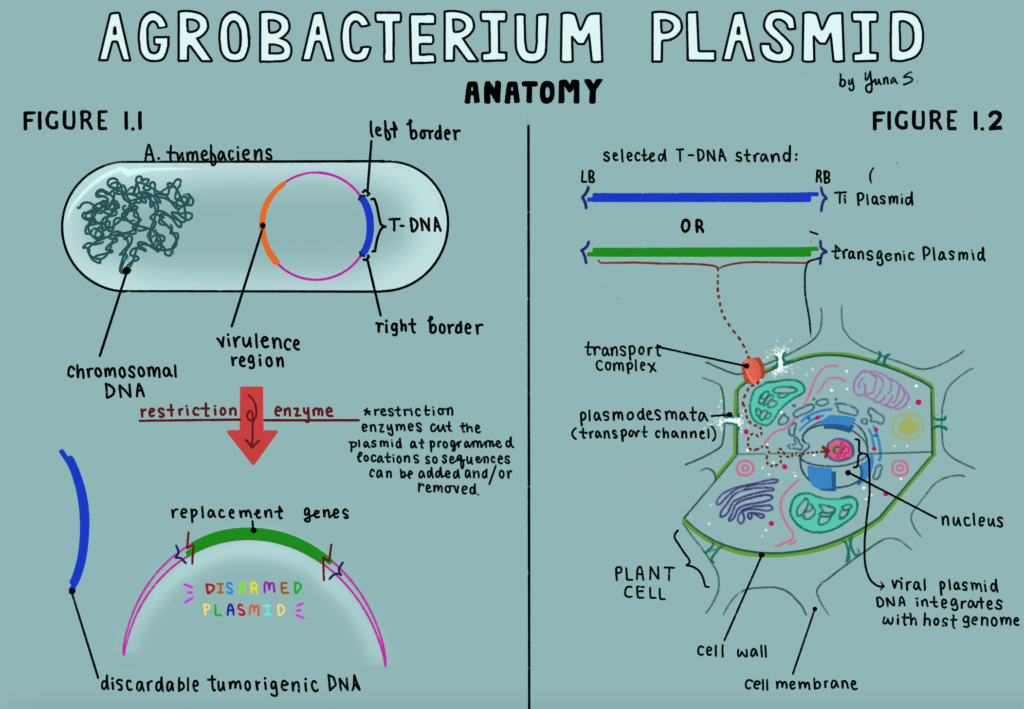
Agrobacterium tumefaciens (not to be confused with my biofertilizer, Azospirillum) is a bacteria you might frequently encounter in nature. While you might not readily recognize it, if you’ve ever seen a tree, bush, or other foliage brandishing an absurd, tumor-looking protrusion on its tissue, you’ve seen Agrobacterium! The microorganism species is a common soil-dwelling microbe that introduces its genetic material through a circular DNA structure called a plasmid (Figure 1.1). These plasmids (specifically described as “Ti Plasmids,” or Tumor Inducing Plasmids, for Agrobacterium) have been a holy grail for plant genetic modification; their natural makeup includes a segment referred to as “transfer DNA,” or T-DNA, that is extracted from the plasmid to infect the host plant nuclei (Figure 1.2). Infection of the plant’s genome is initiated through the release of phenolic compounds (derived/adjacent to phenol, C 6H 5OH), a common stress-signal response, which alert virulence genes to facilitate movement of the tumorigenic T-DNA into the recovering plant.
So… why is Agrobacterium so important for genetic engineering?
Although its typical composition breeds a cancer-like phenotype, the tumor-producing genes within the T-DNA sequence are only a portion of the bacterial genome. They are encapsulated within the plasmid by a left and right border group, which essentially tell the organism to insert everything in between their limits. So when removing the tumorigenic genes, and releasing the left and right borders intact, researchers are left with a mechanism to infect plants without the integration of the tumor growths.
Empty T-DNA are commonly referred to as “disarmed,” and can be programmed by geneticists with replacement genes– any sequence chosen to take the place of the Ti characteristic. These transformed Agrobacterium plasmids can be taken and used to infect a range of host plants with irregular phenotypes, a tool useful for research, agricultural advantages, and medicine (Figure 1.1).
This process is the elected method of Open Plant for their insulin-expressing liverworts, and also of many Cold Spring Harbor PhD students when proving the existence of various biological processes.
Matias is using Agrobacterium to create many mutant plants for his project, so in the next few weeks we will be working with one of his transgenic plasmid constructs to add a fluorescent protein (known as m-scarlet) into the A. Brasilense fertilizer colonies. The ultimate goal is to express both a glowing red pigment and antibiotic resistance in a new generation of bacteria, where the pigment will allow us to document the root colonization during fertilization, and the resistance will act as insurance we can verify the successful implementation of foreign genes. After transforming the Azospirillum, we will grow them on antibiotic agar plates, and the surviving colonies will evidently contain the fluorescent and antibiotic resistance traits, as they will be linked together on the plasmid! (Meanwhile colonies without said genes will not survive due to the presence of an antibiotic, which naturally kills Azospirillum and Agrobacterium.)
Fluorescence Microscopy
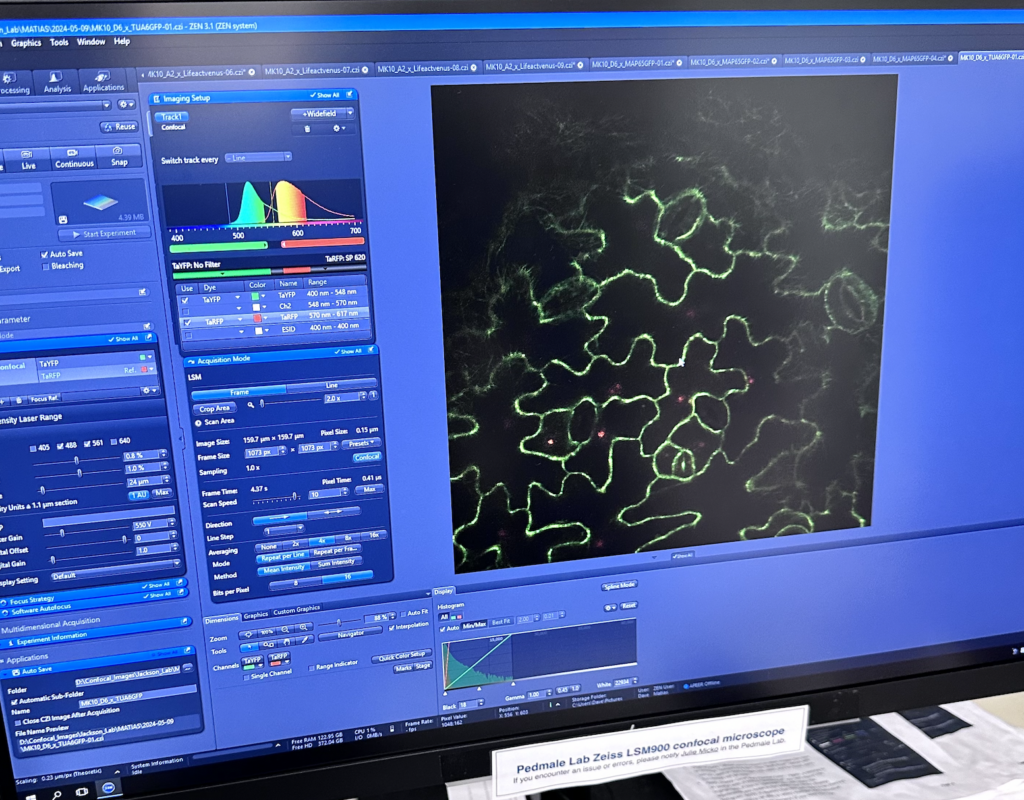
Now, I have an interesting preview for all of you! In the lab this week, Matias already had some samples of his model (Arabidopsis Th., Figure 2) marked with two types of fluorescent proteins: RFP (red fl. protein) and GFP (green fl. protein). These leaf segments were assayed under the CSHL fluorescent microscope, which excites the proteins within the tissue, giving visibility to the movement of cells they are contained in (Figure 3).

LB Plate Colonies
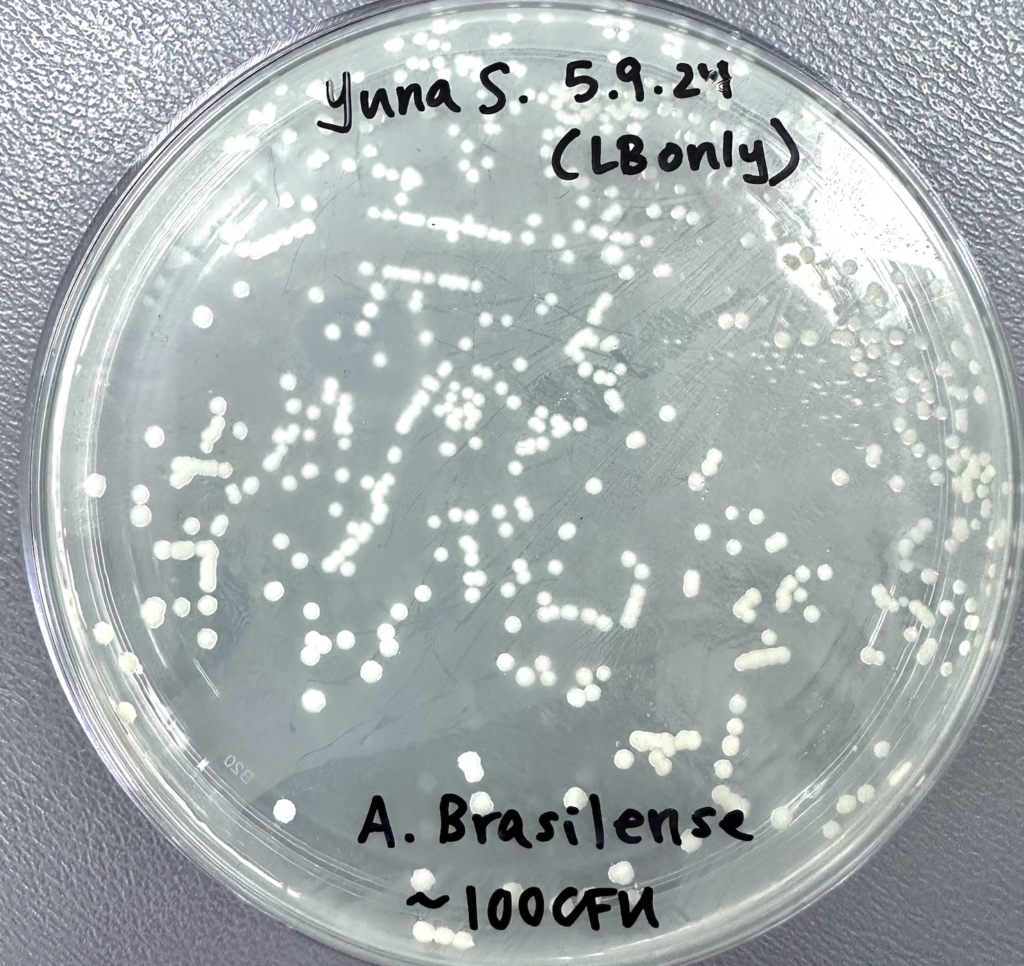
On another note, last week’s Azospirillum plates have finished incubating as of this Thursday! Counting both trials and taking an average of their colonies, I ended up with ~500 colonies instead of 100 (Figure 4). This was perplexing, but not at all a setback in my research, as I’ve documented now that our bacteria are more concentrated than initially thought. However, it was certainly exhilarating to see the emergence of colonies after working with seemingly inorganic powder for so long!
Greenhouse Update:
Enough bacteria talk… Now to the greenhouse progress, where I’m excited to announce that we have Mars growth! After surveying the Earth groups per usual, I detected a small but vigorous sprout shooting up from the Martian simulant insert #16 (Figure 5). What great news! A single biofertilizer-treated seed has sprouted, with hopefully more to follow. If all goes well, we will have happily abundant harvests from the Martian plot in a few weeks. Our sweet ecosystem seems to be adjusting well to the moisture change from last week, and I look forward to updating in stride.

What’s Next?
Along with more greenhouse updates, the upcoming week will include more specifics of our Azospirillum plasmid design, and how Matias and I will adapt the current Agrobacterium strand. Additionally, I intend to sterilize a group of alfalfa seeds so that they can germinate without interference from the MGS-1 simulants, the hope is that root development in a less strained environment will breed a higher rate of post-germination survival.
Cheers!
Reader Interactions
Comments
Leave a Reply
You must be logged in to post a comment.

Yuna- great work this week! I’m so glad that you are seeing harvests in your martian plots. I look forward to checking back to see the rate of post-germination survival of the alfalfa seeds.