Week 8: Gases, Fun, and Azospirillum
May 21, 2024
Greetings, salutations! Welcome to the eighth week of my blog, and thanks for joining me once again.
Recap:
Here’s the ritual breakdown– Last week, we discussed the importance of the soil-dweller Agrobacterium tumefaciens, and the mechanism it uses to transfer its DNA to host organisms. As for hands-on experimental work, I counted some Azospirillum colonies after rehydrating them with distilled water, and discovered a Martian-grown alfalfa sprout, huzzah!
Cold Spring Harbor was beautiful as ever this week, and I’m noticing more and more fruit and flora emerging as spring reaches full throttle. There were even wild strawberry blossoms lining the walkway to Delbruck Lab (The plant research lab at CSHL); life inside was equally active. (Figure 1)

To start off the week, I designed a plan to germinate some alfalfa plants without interference from the harsh regolith (loose rock and dust) environment. The reasoning behind this was to document a visual difference between Azospirillum-inoculated root development and control seeds without the fertilizer; this way, once the alfalfa sprouts are transplanted into their respective soil environments, their early development may provide them with a stronger chance at life.
Chlorine Gas Sterilization:
When picking up the typical household cleaning agent, the fine print of many products may include an important lesson in chemical mixology: always read the safety data sheet. Disclaimers on your average bottle of toilet or tile cleaner will urge against the layering of cleaners and disinfectants for good reason! This is because however efficient it may seem, combining the latent chemical profiles of agents like bleach, cleaners, or dissolvables can form a dangerous cocktail of products– so it’s with great caution that I caution– do not try this at home!
Bleach, also known as Sodium hypochlorite (NaClO), when combined with the compound Hydrogen Chloride (HCl), a common ingredient in many cleaning agents, can form motile chlorine gas (Figure 2). Chlorine gas is highly toxic due to its reactivity as a halogen. Diatomic chlorine gas molecules attack the bonds of water molecules, forming free oxygen as well as HCl, a potent acid able to severely deform cells in the body.
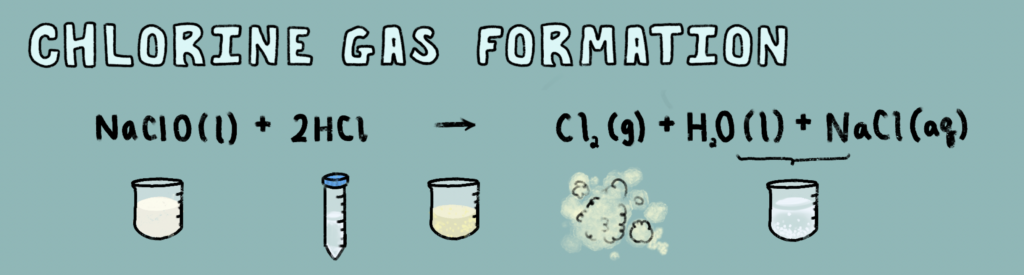
However, while chlorine gas is deadly to people in long-term exposures, it holds quite the advantage in lab sterilization protocols. The reaction between Chlorine and water contains intermediates that penetrate a negatively charged cell membrane in microorganisms, causing premature cell death and reproductive failure in these cells. Because of its efficient disinfecting properties, chlorine gas is used as a sterilizing instrument in many laboratories.
At CSHL, as well as a majority of plant and microbiology laboratories, a popular medium of growing and storing organisms is agar, a seaweed-based solidifying agent that can be supplemented with nutrients or deterrents of many kinds. Its dexterity is a double edged sword, however, because storing a plant without necessary precautions may breed fungus, mold, and bacteria– all which pose risks to the intended organisms longevity. (Figure 3)

Therefore, chlorine gas is a great way to eliminate foreign contaminants when plate-ing seeds, sprouts, and many forms of plants. And it applied favorably to our alfalfa seeds! So on Tuesday, I ran the procedure as follows:
Alfalfa seeds were added to open microfuge tubes and strapped to the edges of a gas chamber. Then, in the fume hood, a beaker containing 50 mL of distilled water, and 50 mL of standard bleach was placed within the center. And to create the Cl gas, 6 mL of HCl is quickly added to the beaker. As the gas forms quickly, I placed the chamber lid on quickly before allowing the gas to sit for 1 – 1.5 hours with the seeds. (Figure 4 – video)
Following the seed sterilization, Cl gasses were evacuated from the chamber and allowed to dissipate, while the neutral product left is an electrolyte solution of table salt (NaCl) and water.
Agar Germination:
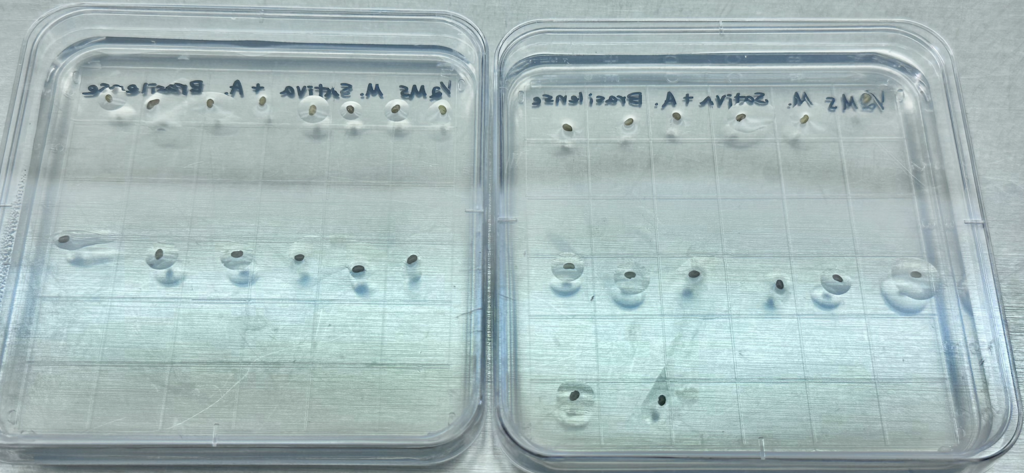
Disinfected seed groups were plated on MS (Murashige and Skoog – a powdered blend of nutrients beneficial for supporting agar plant growth) supplemented agar, using a micropipette and sterile water. (Figure 5). Next week, these plate-germinated seeds will be carefully transplanted into a new plot of Martian soil tests in order to weigh the benefits of isolated germination.
Plasmid Mapping:
On another note, if you are interested in genetic mutations, engineering, or bacteria, I’m thrilled to introduce the key components of our transgenic (containing foreign genetic material) Azospirillum this week! My previous blog articulated the basics of plasmids and genetic transformation, which I will be utilizing to build fluorescent versions of our nitrogen fixing bacteria.
As Azospirillum brasilense does not naturally contain the fluorescence phenotype, this is a factor that must be introduced via genetic alteration. With Matias’s help, I will be combining two of his previously stored plasmid builds into a form receivable by our model bacteria.
To build some background, promoters are sequences of DNA that instruct the cell where (and of what quantity) to send proteins after they are translated from RNA. These sequences are foundational to building sets of DNA because isolating genetic material from one organism requires an agreeable promoter in order to be successfully expressed in another. Allow me to explain:
For example, the gene that encodes for our fluorescent protein of interest is referred to as “mScarlet,” originally sampled and sequenced from a species of coral. Once tissues containing these genes are collected and purified, researchers will have access to the sequence. These lines will contain first the coral-specific promoter, and downstream the gene itself (Figure 6).
However, in the instance that someone (for example, me) wanted to express that same gene within a bacteria (for example, Azospirillum), the coral-specific promoter would be largely unfit when attached to the mScarlet gene. Because there are vast anatomical differences between corals and bacteria, if a coral-promoter-containing plasmid was added into bacteria, the promoter would essentially tell the cells “send a lot of this protein to the polyps and tentacles,” to virtually no effect, because bacteria do not have polyps and tentacles. This short circuit imposes the need for geneticists to split the sequence and discard (or save for later) the original promoter, and add a promoter fit to the model organism.
This is what we are doing! MScarlet is our exact protein of interest, providing the brightest red fluorescent pigment to date! And in the coming weeks, I will be inserting the promoter from a bacterial plasmid (Figure 7, right) into a construct containing the saturated FP (fluorescent protein – Figure 7, left).
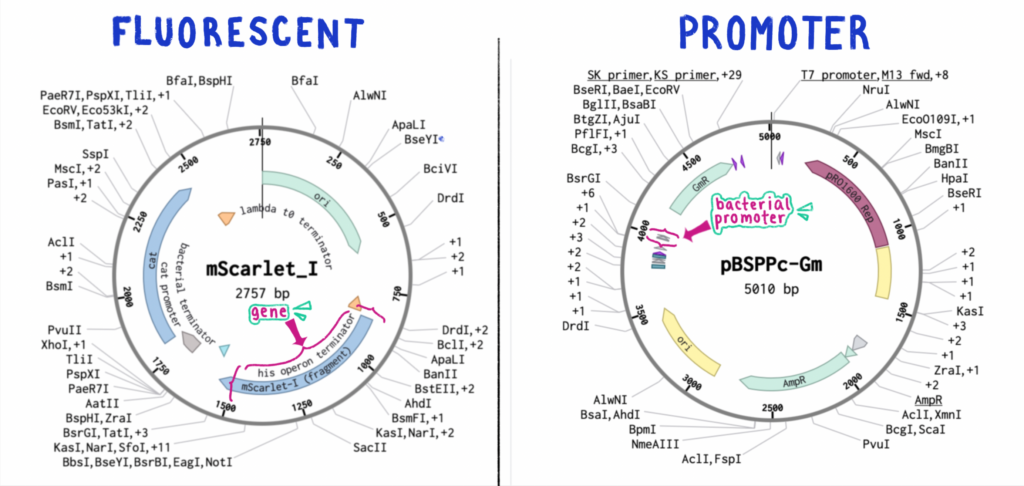
The bacterial promoter we intend to extract is from the plasmid created in Gabriele Labes’s “Isolation and characterization of a strong promoter element from the Streptomyces ghanaensis phage I19 using the gentamicin resistance gene (aacC1) of Tn1696 as reporter,” and is referred to as the pc promoter.
Greenhouse update:
Back to the plants!–
Once again, it’s time for bacterial treatments. Following the procedure outlined in Week 6, I added the same stock solution of Azospirillum to replenish the bacteria soil groups.
As for growth checks, the earth control groups are demonstrating clear differences in both height and biomass production when at different levels of biofertilizer. In Figure 8, the non-treated Earth group (left) is much smaller than the treated Earth group (right). These controls can help demonstrate the effectiveness of our Azospirillum for improved vigor in the final research article.

Unfortunately, no new sprouts have emerged from the MGS-1 simulants (Figure 9). The single stalk from Week 7 is still going strong, but if the lack of germination persists I intend to outsource my experimental design in order to produce a better sample size for the final biomass weigh-in and tests.

What’s Next?
My next line of action includes planting the germinated seed groups in our MGS-1 regolith, and Chlorine-sterilizing another group of seeds to keep for colonizing with our fluorescent Azospirillum. Further, we will begin modifying the MScarlet plasmid to be compatible with our bacteria, and a stock solution of electrocompetent (ooh!… what’s this!..) cells will be frozen for DNA insertion.
Thanks for joining me, and stay tuned!

Leave a Reply
You must be logged in to post a comment.