Week 6: Building reconstituted embryonic mouse ovaries (rOvaries) in culture
April 11, 2024
Bonjour tout le monde! I’m back from my week in Europe, where I got to eat Swiss chocolates in Zurich, see the Eiffel Tower in Paris, and visit Platform 9 ¾ in London (unfortunately, I couldn’t go through the wall 💔)
Back into it! This week is probably the most important week of my project. Our goal was to build reconstituted ovaries of different sizes in culture. Or more specifically, reconstituted embryonic mouse ovaries (as we attempted in Week 5)! Creating ovaries in culture allows us to observe oocyte formation and attrition and follicle production, in addition to generating “an alternative source of gametes for reproduction” which will further fertility medicine and research (Hayashi et al., 2017). To do this, we needed to once again dissect the pregnant mouse, gather her embryos, and dissect the female embryos to retrieve the embryonic ovaries (as remember, my project deals with studying germ cell migration, proliferation, and selection during embryonic development!). After dissociating the ovaries, we put them through a FACS sorting machine so we can gather the germ and somatic cells! As a reminder, germ cells (which are at the oogonia stage at our dissection timepoint) express a green fluorescent protein (GFP) that the sorting machine can detect, which helps it distinguish between germ and somatic cells. We also unfroze banked somatic cells, since the ratio of somatic to germ cells is very high, and somatic cells can survive well being frozen and thawed.
Now that we have the oogonia and somatic cells that compose ovaries, we did a LOT of math to get them at the concentrations in media that we wanted (as in how many thousands of cells per mL). Then, using our fixed concentrations, we took out different amounts of our cell solutions for each oogonia and somatic cells and put them together in a 96-well plate to begin creating ovary replicates of different sizes. The protocol we referenced used a ratio of 5000 germ cells to 50000 somatic cells when putting cells on the plate. Our goal was to see how much we could scale this protocol down for the reconstitution to still work. We made a replicate of the same size as the protocol (5k), a replicate of 3000 germ cells to 30000 somatic cells (3k), and 3 replicates of 1000 germ cells to 10000 somatic cells (1k).
The next day we came in to image the rOvaries and check how they aggregated. After a lot of imaging, we were able to see that it seems to have worked! The somatic cells (which included pre-granulosa cells, cells that support the oocyte and provide nutrients) were aggregating and proliferating. The oocytes were also visible due to their fluorescence under the microscope! However, one of the 1k replicates did not seem to have aggregated much at all, so going forward we will only be using the 2 1k replicates that survived.
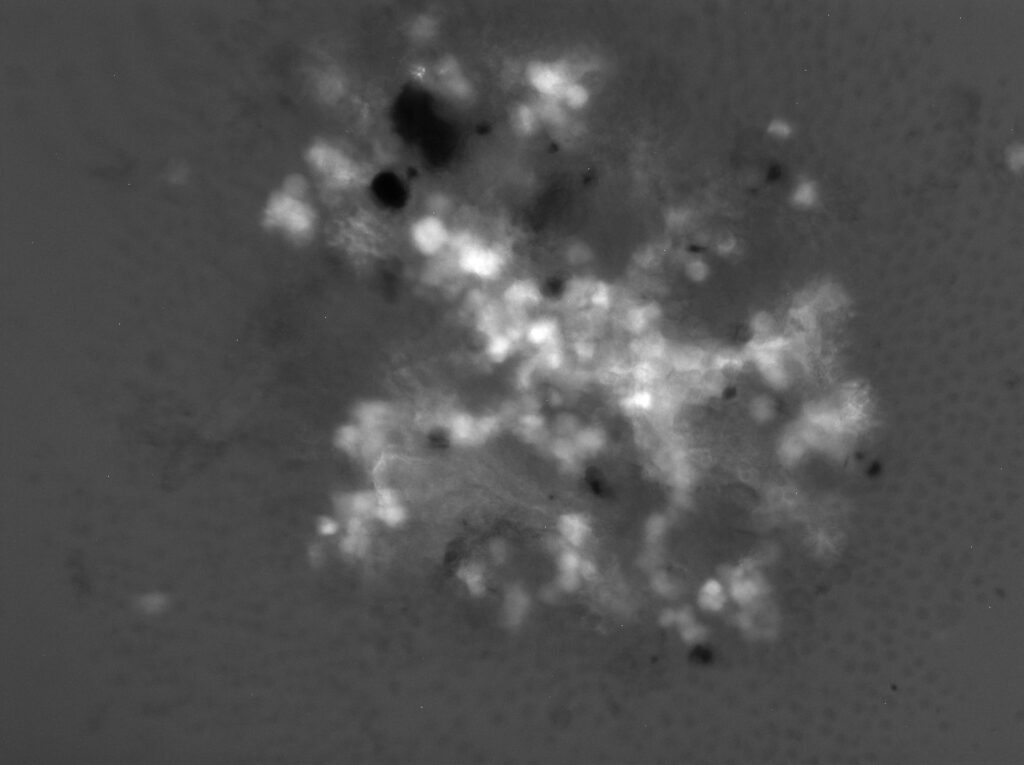
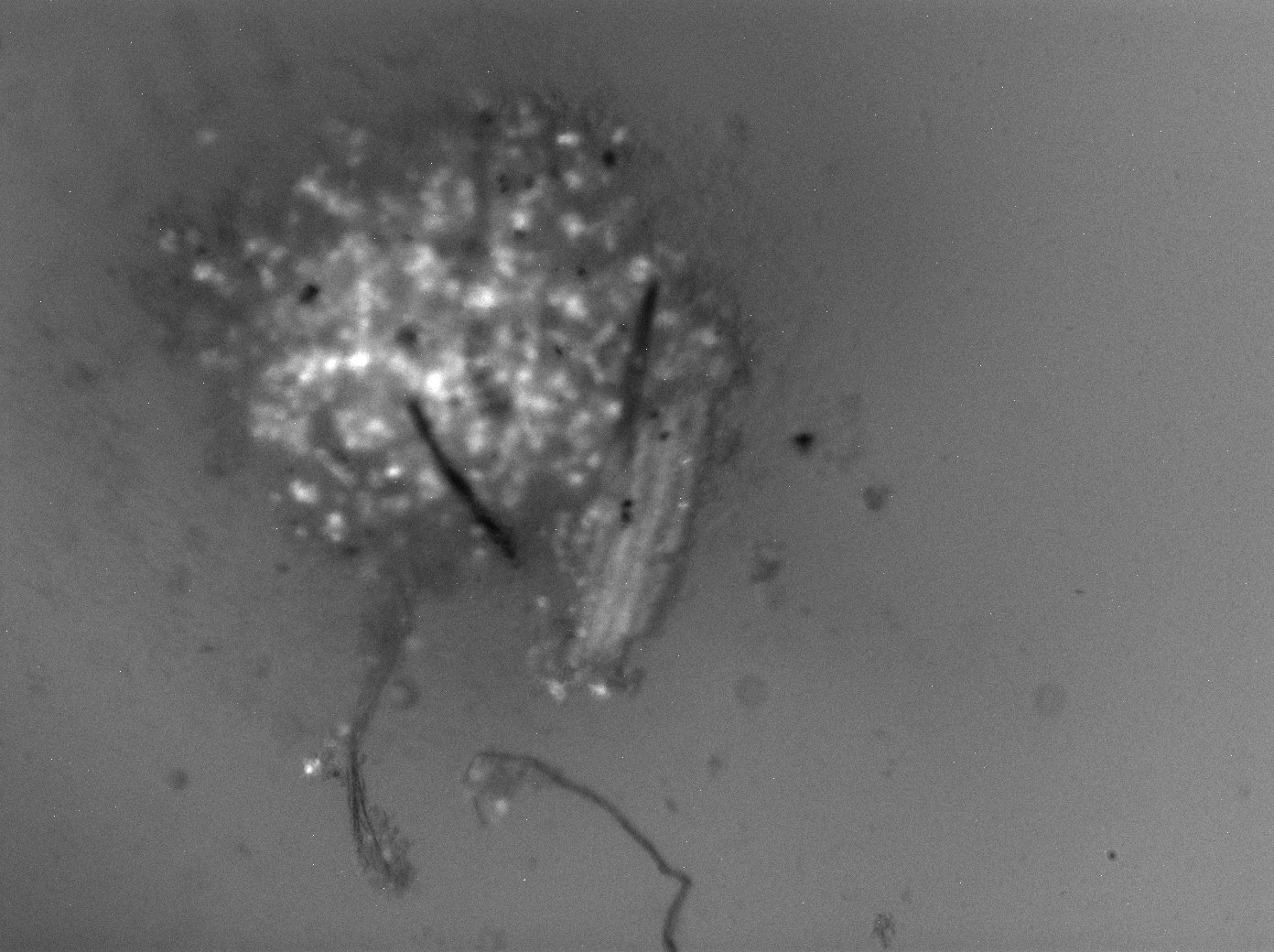
When imaging the rOvaries, I image them 4 ways – under 4x and 10x magnification, under each of two lasers: brightfield (standard) and the 488 laser. 488 nm is the wavelength for green light, so it will help illuminate the cells that Oct4GFP (remember GFP = green fluorescence protein, and the cells that express them are the oogonia!)
I’ve attached a sample of the images I have been generating! All the images show the rOvaries after 1 day in culture. The first image is one of the 1k rOvary replicates at 10x magnification, the second image is the 3k rOvary at 4x magnification, while the 3rd image is the 5k rOvary at 4x magnification. This week, I am attaching pictures of what the rOvaries look like under the 488 laser. Next week, I will show them under the brightfield laser! Notice the glowing dots on the images – those are collections of oogonia. As they mature into oocytes, the oogonia will express less and less Oct4GFP, but as of right now, we expect the strong GFP expression.

On Monday, I also got to watch another dissection – but this time, it was a mouse brain dissection done by another lab’s team member.
That’s it for me! Anjali out.
Reader Interactions
Comments
Leave a Reply
You must be logged in to post a comment.

This is wonderful Anjali! Great job! This research experience will be unforgettable. Keep up the good work.
By the way.. I didnt know that you went to Europe. I am jealous! 🙂